|
To add a new MSA study, click on the arrow next to the  button on the main screen toolbar and select the study that will be performed. button on the main screen toolbar and select the study that will be performed.
To edit a previously created study, select it in the list of records and click on the  button. button.
▪MSA planning is similar to all types of studies. For that reason, both will be described in this section and identified where they differ. ▪Refer to the Studies section to check the description of the different studies provided by the MSA. |
Fill in the following fields on the data screen that will be displayed:
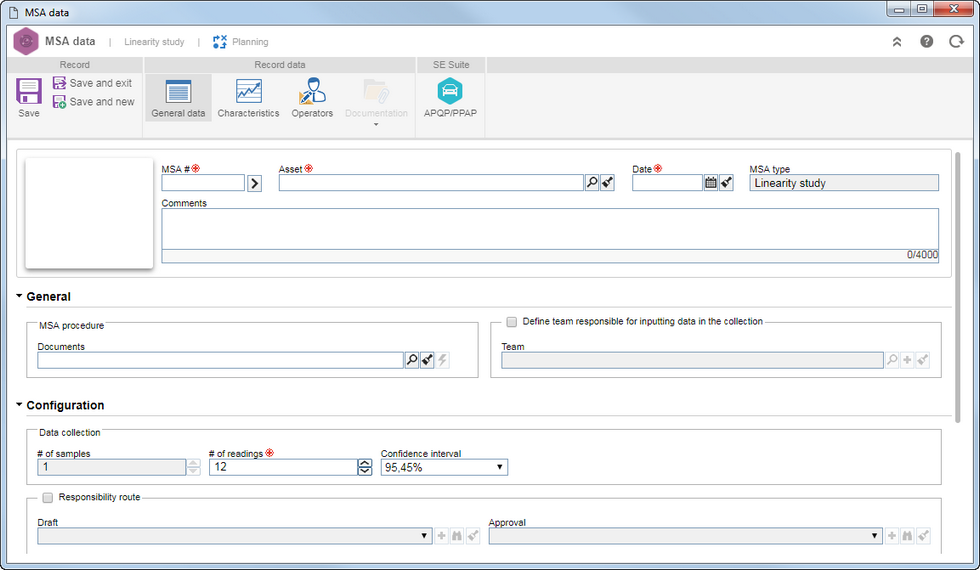
MSA #: Enter the number or code that identifies the MSA. Use the arrow next to this field to generate an automatic ID #. Do not forget that if on the general parameters screen is configured a mask to be used, upon generating the automatic ID #, the system will display the respective mask. This field can be disabled if the configuration is also done through the general parameters screen.
Asset: Select the asset to be analyzed. All assets with Gage specification, where the user is part of the responsible team will be displayed in the search of this field.
Date: Enter the planning date. This field is automatically filled by the system and displays the current date, but it can be edited.
MSA type: This field displays the MSA type to be planned.
Comments: Use this field to enter the MSA comments.
Then, fill out the fields in the following sections:
Note: Some sections will only be available after the record has been saved.
General data
General
|
|
MSA procedure
|
Select the document required for the measurement system analysis. If SE MSA is integrated with SE Document, the system will allow associating documents from the latter. Remember that the integration between SE MSA and SE Document should be made through the general parameters screen.
|
Define team responsible for inputting data in the collection
|
Use this field to select a Team that is responsible for entering the collection values in the system. The collection is performed by the operator, but data related to it can be input by another user. In that case, define the team that will do it. Remember that the team record is managed through the SE PDM component.
|
Configuration
|
Determine the measurement system analysis configurations:
|
# of samples
|
Enter the number of samples of the part to be analyzed. For Linearity and Independent sample studies, this field cannot be changed.
|
# of readings
|
Enter the number of readings that will be performed in each sample. This field does not appear in the Analytic method and cannot be changed in the Range study.
|
Confidence interval
|
Enter the expected range of values containing, within a desired probability called "confidence level", the true value of a parameter. Remember that depending on the study chosen by the user, this field will not display.
|
R&R results
|
This field is only available for the RR (average and range/anova) study. Thus, define how to calculate the percentages of the study:
▪TV: Total variation of the study. It is calculated by the square root of the sum of the repeatability and reproducibility (R&R) and part variance (PV) squared. ▪Tolerance: Deviation allowed for a standard or nominal value that still maintains the desired setting, form and function. Enter the tolerance value in the respective field. |
Approval criteria (R&R)
|
This field is only available for the R&R (Average and Range/Anova) study. The results must be evaluated to determine if the measuring gage is acceptable for its intended application. A measurement system must be stable before any additional analysis is considered to be valid. Enter the approval and rejection percentage of the process.
|
Responsibility route
|
Check this field so that the subject MSA plan goes through the draft and approval steps. In that case, using the respective fields, select the responsibility route that will draft and approve the MSA.
|
Note: The "# of samples", "# of readings" and "Confidence interval" fields can be disabled if the respective study has data already defined, according to ISO/TS 16949 Standard.
|
Attributes
|
See all the attributes that were associated with the MSA study through the Configuration  General parameters menu. If there are no associated attributes for the MSA study in question, this section will not be displayed. General parameters menu. If there are no associated attributes for the MSA study in question, this section will not be displayed.
Thus, those that are required must have their values filled out. To do that, input them in the Value column in the attributes list. However, the value selection/filling out mode varies according to the configuration set for each attribute.
|
Characteristics
In the Characteristics section, the characteristics of the part to be analyzed should be entered. To do that, click on the  button in this section to fill in the following fields on the screen that will be displayed: button in this section to fill in the following fields on the screen that will be displayed:
i.
|
Item: Select the item (part) to be measured. Remember that the possible items should be recorded in SE PDM component.
Revision: Select the revision of the item (part) to be measured.
Characteristic: Select the characteristic of the item (part) to be measured.
Process variation: Input process variation, that is, the standard deviation or difference between USL characteristic (Upper Specified Limit) and LSL (Lower Specified Limit) of the characteristic. This field will not be displayed for Cross tabulation and Signal detection methods.
Tolerance: This field will only be submitted for the Signal detection method for the process tolerance to be informed.
|
ii.
|
Fill out the fields and then click on Save. At this point, the characteristic will be associated. If necessary, repeat the procedure to associate more characteristics.
|
Some studies, such as Linearity, require more than one characteristic to be associated so that MSA plan can go to the collection step.
|
|
Operators
In the Operators section, operators of the gage that will measure the part(s) are associated. To do that, click on the  button of this section. At this point, a selection screen will be displayed. Enter the operator and click on Save. According to the STANDARD and depending on the subject study, you must select only 1 (one) operator or more than 1 (one). button of this section. At this point, a selection screen will be displayed. Enter the operator and click on Save. According to the STANDARD and depending on the subject study, you must select only 1 (one) operator or more than 1 (one).
|
Actual status
The Actual status section will only be displayed for the following study methods: Details, Signal detection and Cross tabulation. In that case, the appropriate markups may be made on the actual status of the part. See how to proceed according to the MSA study type:
▪Cross tabulation method: Each one of the operators must give his/her opinion on the status of each sample of the part. When the status is checked, it shows that the part conforms to specifications. ▪Signal Detection method/Analytic method: Each operator must enter the reference value of each sample of the part in the respective field.
|
Documentation
Attachment
|
It is possible to add attachments related to the MSA planning. For further details on how to associate attachments with the record, see the Add attachments section.
|
Document
|
It is possible to associate documents from the SE Document component related to the MSA that is being created. Remember that the integration between SE MSA and SE Document is configured through the MSA general parameters. Refer to the Add documents section for further details on how to associate documents from SE Document.
|
|
APQP/PPAP
In the APQP/PPAP section, it is possible to verify the APQP/PPAPs associated with the MSA in question. Select the desired record and click on the  button to view the data. button to view the data.
Refer to the SE APQP/PPAP documentation for further details on how to perform the association.
For this feature to work correctly, the SE APQP/PPAP component must have an active license key in SE Suite.
|
|
Save the record. At this point, the system will ask whether you wish to send the record to the next step. Choose the desired option:
OK: If there is no inconsistency in the MSA plan data, the record goes to the MSA drafting step, if that has been defined in the approval route, or to the measurement data collections.
Cancel: The record will remain with the "Planning" status.
|





